Scientific abstract
The spinocerebellar ataxias (SCAs) are a genetically heterogeneous group of autosomal dominantly inherited disorders that are clinically characterized by progressive loss of balance and coordination. Brain pathology of SCAs centers around the cerebellum and brainstem. The most common SCAs (SCA1, SCA2, SCA3, and SCA6) are caused by instable expansions of polyglutamine encoding CAG repeats which led to the designation of these diseases as polyglutamine SCAs. Based on a greatly increased understanding of the pathobiology of polyglutamine SCAs, new treatments that aim at silencing the disease genes are moving towards the phase of clinical trials. These approaches are particularly promising, if applied as preventive therapies in presymptomatic mutation carriers. For any trial, whether therapeutic or preventive, meaningful and sensitive biomarkers that reflect disease state and severity are of critical importance. Cerebellar and brainstem regional volumes derived from 3T MRI studies are useful tools to assess SCA-related alterations, but are in no way sufficient in view of the challenges to the required specificity and sensitivity of clinical biomarkers in upcoming clinical trials. Ultra-high field MRI (UHF-MRI) has an enormous potential to detect and monitor structural and chemical brain changes at an unprecedented level of detail that cannot be achieved with 3T. However, technical shortcomings caused by field inhomogeneities at 7T that degrade image quality of the cerebellum and brainstem have so far prevented the exploitation of UHF-MRI to study SCAs. Overcoming these technical challenges by utilizing novel parallel transmission (pTx) MRI technology, the application of quantitative MRI at 7T in SCAs will mark a breakthrough with direct translational impact for SCAs.
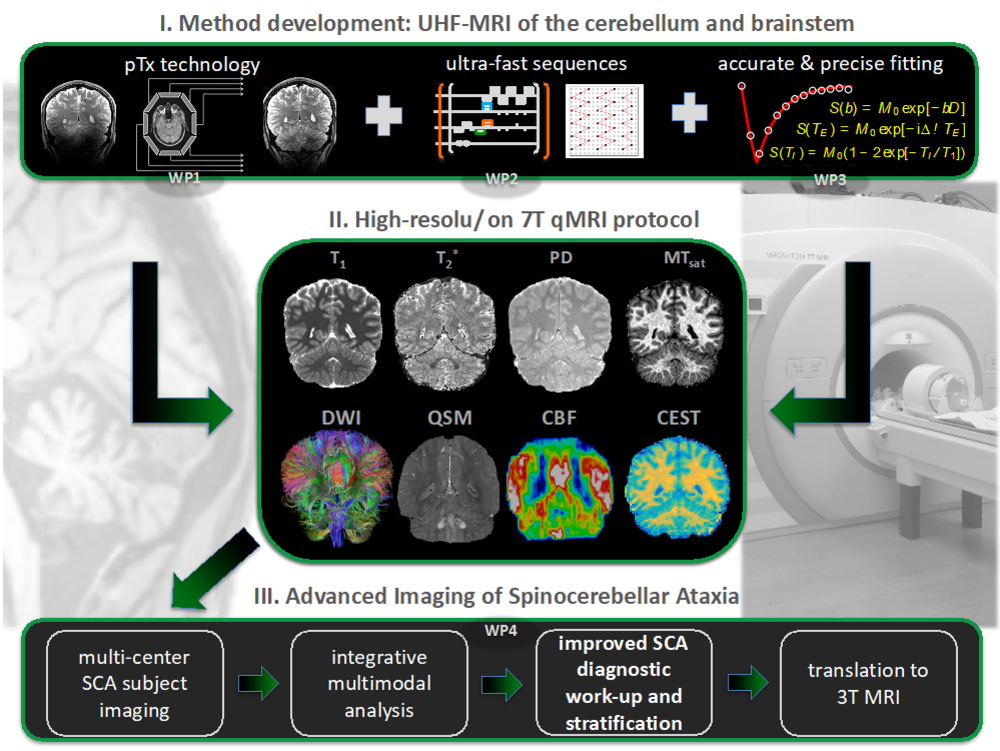
Our goal is to establish quantitative UHF-MRI biomarkers for polyglutamine SCAs with high potential for detecting early disease manifestation and monitoring progression. High-resolution multi parametric mapping (MPM) of brain structure is explored, which provides estimates of MR relaxation times (T1 & T2*), proton density (PD) and magnetization transfer saturation (MTsat). Additionally, diffusion weighted imaging (DWI) is utilized to quantify brain connectivity and tissue microstructure and quantitative susceptibility mapping (QSM) to estimate tissue iron load. Finally, brain metabolism is explored with molecular imaging, namely MR spectroscopic imaging (MRSI) and chemical exchange saturation transfer (CEST) imaging. All sequences are tailored for high-quality imaging of the whole brain, including cerebellum and brainstem, and for convenient application in patients. The UHF-specific transmit field inhomogeneities, which are specifically challenging in the cerebellum and brainstem, are mitigated with novel pTx technology. Additionally, fast imaging readouts enable the acquisition of all contrasts. The short and patient-friendly MR-protocol is currently tested in a pilot study at the 7T systems of the contributing partners.
The project partners contribute complementary expertise: the MR Physics group at DZNE (Bonn) implements fast quantitative UHF-MRI sequences, which utilize the pTx technology jointly developed by DZNE, NTNU (Trondheim) and Bilkent University (Ankara), while data analysis and parameter mapping is developed by GIGA-CRC (Liege). A multicenter study involving all participating sites that applies the new UHF-MRI sequences is coordinated by DZNE Clinical Research (Bonn).

