Clinical Research Platform
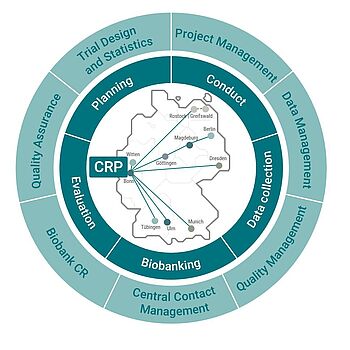
The multi-site studies of the DZNE are supported and coordinated by the Clinical Research Platform (CRP) at the Centre for Clinical Research in Bonn. Within the clinical research of the DZNE, the CRP provides an organizational framework for the planning, execution and evaluation of clinical trials as well as the collection of all data and biobanking under standardized conditions. The CRP comprises the areas of project management, data management, trial design and statistics, central contact management, clinical research biorepository, quality assurance and quality management. The study database is operated in cooperation with DZNE-IT. The composition of the CRP team of experienced scientific, clinical and industrial staff ensures a high degree of quality, transparency and consistency of clinical studies from conception to completion.
The quality management system introduced for the CRP was successfully certified for the first time in September 2017 and successfully re-certified in September 2020 after the extension by the trial design and statistics area. In January 2023, the CRP and the Clinical Trials Unit (CTU) at the Bonn site were merged into a joint quality management system and successfully re-certified in accordance with DIN EN ISO 9001:2015 in October 2023.
Biobank of the Clinical Research
The clinical research of the DZNE collects human biomaterials in mono- and multicenter studies. The biomaterial is stored in the biobank of clinical research and made available to researchers on request to answer specific questions posed by research projects. The biomaterial samples sent by the clinical research study centers are received, stored and, if necessary, processed further. The biobank is responsible for certain types of processing of the biomaterial, such as the further processing of CPT whole blood to obtain PBMCs and ensures a consistent quality of the biomaterial.
Data Management of the Clinical Research
Data management covers technical processes for data collection, data administration and data use, with particular attention to internal and external quality requirements as well as legal regulations (national and international), guidelines and recommendations. Data collection is carried out on a decentralized basis at the individual centers, while data management and the export of data for research purposes are centrally controlled by the DZNE.
Project Management of the Clinical Reserarch
Clinical studies at the DZNE are conducted both monocentrically and multicentrically at the different sites of the DZNE. The project management team of Clinical Research supports the principle investigators from the planning of the study to the preparation, initiation and coordination of the individual study centers. Project management is the interface between all persons and departments involved in conducting the study (Data Management, Quality Assurance, Principal Investigator, Assistants, Laboratory, Imaging, Administration, etc.).
Quality Management
Quality management supports both the Clinical Research Platform and Clinical Trials Unit at the Bonn site as well as the entire Clinical Research of the DZNE in the standardization, definition and documentation of quality-relevant processes This also includes the structured instruction and training of their employess and study teams with the help of an e-learning platform, which is also used by industrial cooperation partners. Audits, both internally as well as in the study centers and with contract partners, contribute to the continuous improvement of the processes. By involving all employees of the Clinical Research Platform and the clinical trials unit, the implementation, maintenance and continuous improvement of all quality-relevant processes of the quality management system and thus a high quality and reliability of the study data is ensured.
The quality management system introduced for the CRP was certified for the first time in September 2017, extended to include the Clinical Trial Unit (CTU) at the Bonn site in January 2023 as part of a joint certification, and successfully re-certified in accordance with DIN EN ISO 9001:2015 in October 2023.
Quality Assurance of clinical studies
Quality assurance checks in the forefront of a study whether all legal and regulatory requirements for the conduct of clinical studies are fulfilled and supports the respective study management in fulfilling these requirements. It is in close contact with the DZNE's Legal Affairs and Data Protection staff units. A key focus here is to ensure that the safety and rights of study participants are guaranteed as well as to review applications for the transfer of data and biomaterial within the framework of research projects and to pass them on to the relevant committees.
A prerequisite for the review of a planned study for compliance with all regulatory requirements is the registration of the study by submitting the "Human Studies“ form via klinische-studien(at)dzne.de.
In clinical studies, the Clinical Monitor monitors the progress of the study and the conduct, documentation and reporting in accordance with the study protocol, Standard Operating Procedures (SOPs), Good Clinical Practice (ICH-GCP) as well as compliance with applicable regulatory requirements and the protection of the rights and well-being of subjects. This includes, among other things, the verification of the qualification of the study group members, the available resources and facilities, the handling of trial drugs as well as the verification of all written consent declarations, the reported data on the basis of the original documents (source data verification). On-site monitoring ensures that the participating trial centres have all up-to-date documentation and testing materials available that are necessary for the proper conduct and compliance with legal regulations.
Trial Design and Statistics
In cooperation with the Institute of Medical Biometry, Informatics and Epidemiology (IMBIE) of the University of Bonn the clinical research platform offers support for both clinical trials and strictly scientific projects to the clinical researchers of the DZNE. The approach is to translate scientific problems into realistic study designs with statistically verifiable hypotheses. This is done by determining clinically relevant results, estimating the sample size and statistically evaluating the study data using a statistical analysis plan. The entire portfolio ranges from statistical advice to statistical reports.
Central Contact Management
The central contact management is provided by the CRP's team assistance, in which it initiates the processes as a contact point for study participants, with which subjects can assert their rights to information, data transfer, blocking or deletion of data or destruction of samples (in accordance with the European General Data Protection Regulation (GDPR)).