ABCD
Evaluation of the efficacy and safety of Rituximab in patients with Amyotrophic Lateral Sclerosis (ALS)
A Randomized, Double-blind, Placebo controlled Pilot Study on B-Cell Depletion as treatment of patients with Amyotrophic Lateral Sclerosis (ALS)
This trial is a multicenter, interventional, randomized, double-blind, placebo-controlled pilot study in parallel design phase IIa. The objective of this clinical trial is to investigate the impact of rituximab (Rixathon®) on the disease progression of patients with amyotrophic lateral sclerosis (ALS). The study is being led by Prof. Dr. Harald Prüß, DZNE and Charité Berlin, and is being conducted at two trial centers in Germany - Charité Berlin and DZNE Bonn. The study is funded by the German Federal Ministry of Education and Research (BMBF) (grant number: 01KG2124).
Background
So far, there is no effective therapy against ALS. Moreover, the causes of the disease have not yet been fully understood. The only drug currently available in Germany for ALS, riluzole, has an average life-prolonging effect of 2-3 months. High hopes were placed in Edavarone, another ALS drug, which unfortunately have not been fulfilled in current trials.
Despite research efforts, ALS is still a fatal disease for which there is no effective treatment available. The current trial focuses on the role of the immune system in ALS, which may be much more responsible for nerve cell death than previously thought. If this trial shows that rituximab leads to a deceleration of disease progression, it would offer new therapeutic options for ALS patients. In addition, the trial may improve understanding of disease progression and, in the long term, contribute to the development of other drugs for ALS, as well as for other neurodegenerative diseases. Rituximab is a drug undergoing clinical testing, which means that it has not yet been approved by the authorities for the treatment of ALS. However, it has been approved in Germany since 1997 for the treatment of non-Hodgkin's lymphoma. In the meantime, rituximab is used for several other diseases and is therefore well evaluated. In addition to the treatment of cancer, rituximab is used for autoimmune diseases.
Overview
The study will include 52 subjects with a clinically definite or probable diagnosis of ALS. In the treatment arm, participants will receive a total of four infusions of rituximab, in the placebo arm, they will receive an infusion with saline solution.
Course of the study
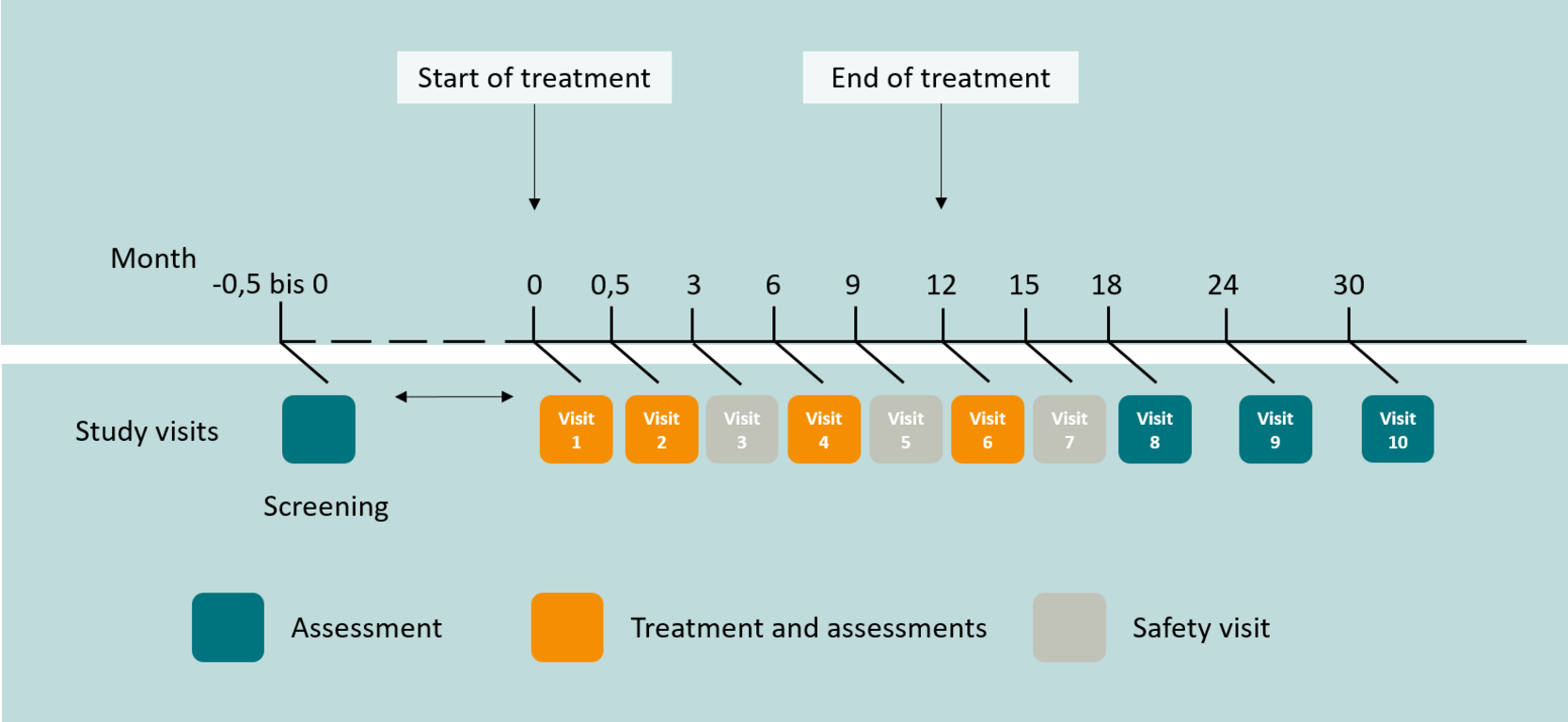
Subjects will participate in the study for a total of 30 months after the initial visit. Over a period of 12 months, participants will receive the drug to be tested or the placebo four times as an infusion at the study center and will additionally attend three safety visits. To assess the long-term effects of the drug, three on-site follow-up visits at 6-month intervals following treatment will take place. Subjects will attend a total of 11 visits (including pre-assessment) at the trial center. During the course of the trial, two lumbar punctures, eleven blood draws and one ECG will be performed.
Principle Investigator: Prof. Dr. Harald Prüß
Start of the study: June 2023
Status: multi centric, study under preparation
Study coordination
If you would like to participate in this study, please contact the
Medical coordinator (DZNE Berlin):
Dr. Rosa Rößling
rosa.roessling(at)charite.de

