Research areas/focus
All animals form memories to adapt their behavior in a context-dependent manner. With increasing age, however, forming new memories becomes less efficient. While synaptic plasticity promotes memory formation, the etiology of age-induced memory formation (AMI) remained enigmatic. To overcome the challenges associated with disentangling causative factors of AMI from adaptive/protective changes during aging, the Sigrist group uses an olfactory conditioning paradigm in Drosophila. This allows them to combine mechanistic analysis of highly conserved neuronal circuit elements with an efficient genetic and cell biological experimental access for studying aging and memory.
Previous work of the Sigrist lab showed that pharmacological and genetic restoration of autophagy protected from age-induced memory impairment in Drosophila (Gupta et al., 2013). In addition, they recently showed that restoration of autophagy operates directly at the synaptic level and kept synapses in an operational space compatible with forming long-term memories fighting the normally occurring up-shift of presynaptic structure and function (Gupta et al., 2016). How mechanistically autophagic regulations intersect with synaptic plasticity mechanisms, however, remains an interesting but unsolved question. Their preliminary results now implicate the evolutionary highly conserved NPY-signaling in communicating in between the autophagic status and synaptic plasticity in a non-cell autonomous fashion.
They find that i) genetic impairment of autophagy already in young animals reproduces the synaptic changes normally typical for aged animals, ii) that defective autophagy reduces NPY-signaling and iii) that genetic elimination of NPY-signaling reproduces the age-typical synaptic phenotypes in young animals. Notably, age-modulation of hypothalamic NPY-signaling is suspected to contribute to age- associated learning and cognitive deficits. Thus, our results identify NPY-signaling, connecting autophagic signaling with a brain-wide synaptic metaplasticity event gating memory formation, as a promising spot of therapeutic intervention to ameliorate AMI. Future work also in rodent models together with the Schmitz and Garner groups will seek to advance the mechanistic understanding of AMI. In preparation of these efforts, we recently characterize presynaptic signaling in rodents together with the Schmitz lab (Grauel et al., 2016).
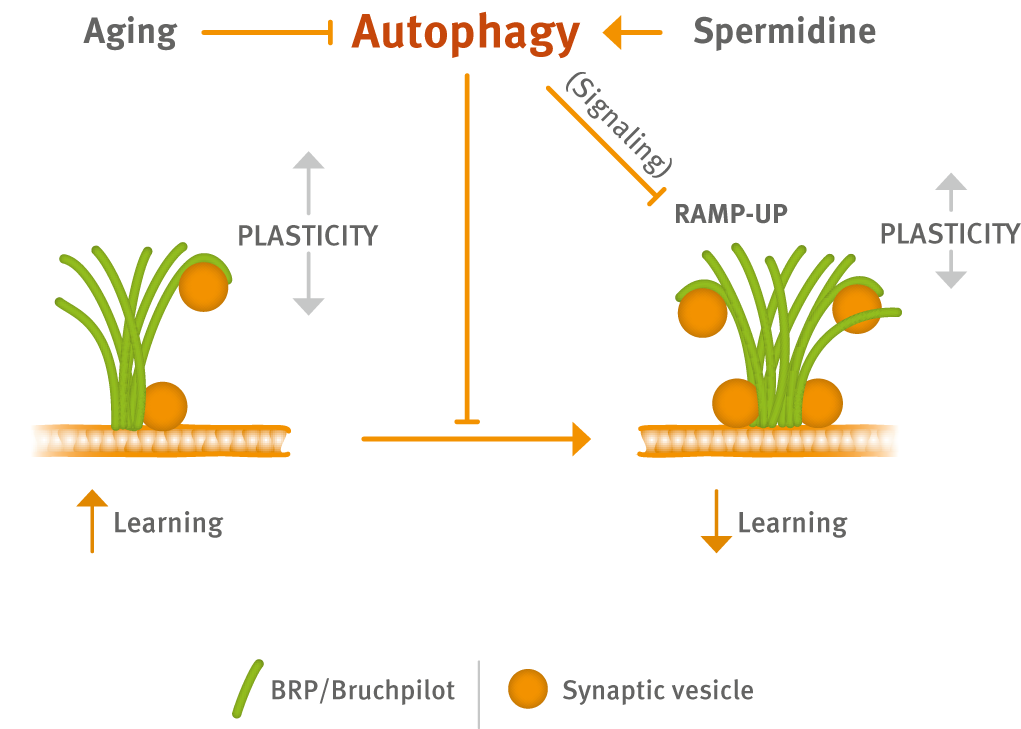
Recently, the group provides evidence that Spermidine boosts autophagy to protect from age-associated upshift in the ultrastructural size and release function of the presynaptic active zone (Fig. 1) (Bhukel et al., 2019).