Areas of investigation/research focus

The zebrafish is a small tropical freshwater fish, which is genetically amenable. Zebrafish are easily bred in a laboratory environment and each female can give hundreds of eggs each week. The embryos and larvae are transparent ideally suited to identify gene function, perform non-invasive in vivo imaging, and drug testing. The research group Zebrafish Models is using genetic Zebrafish models to identify gene function and to study molecular mechanisms of the neurodegenerative diseases Alzheimer disease (AD), amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD). Genetic zebrafish models are generated by cutting-edge CRISPR/Cas9 genome editing technology to generate knock-out animals, introduce disease-associated mutations, or modify endogenous proteins. The Zebrafish Models group was one of the first groups applying this technology to modify the zebrafish genome (Hruscha et al., Development 2015). The main focus of the Zebrafish Models group is the elucidation of the physiological and pathophysiological functions of RNA-binding proteins in ALS and the physiological function of AD-associated proteins BACE 1 and -2 and the Amyloid Precursor Protein (APP).
By running a large modern state-of-art zebrafish facility we further provide a technology platform and knowhow for collaborating research groups within the DZNE.
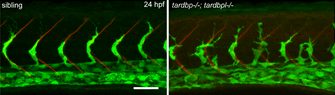
The transparent zebrafish larvae enable us to visualize what is happening inside the organism non-invasively. By labeling different cell types we can visualize the interaction between these cell types in vivo. In the example below, we visualize the vasculature in a live fish with a green fluorescent reporter and are able to follow the developing vasculature.
Zebrafish that lack functional TDP-43 (a gene mutated and aggregated in almost all cases of amyotrophic lateral sclerosis) show a dramatic mis-patterning of the vasculature (shown in green - on the right in mutants compared to wildtype on the left). The analysis of the TDP-43 mutant fish uncovered this very unexpected link between neurodegeneration and the vasculature. The molecular mechanism leading to vascular defects have been elucidated and were also affected in ALS patients. These targets of TDP-43 function might provide a suitable alternative therapeutic strategy to treat ALS.
By studying disease gene function and the consequences of mutations in disease genes the Zebrafish Models group contribute in the general understanding of the molecular mechanisms of disease.