Areas of investigation/research focus
Neurotransmission relies on the tight spatial and temporal regulation of the synaptic vesicle (SV) cycle. The average nerve terminal contains large amounts of presynaptic proteins, above 100 mg/ml according to quantitative analyses, and SVs, mitochondria and other organelles occupy nearly half of the volume of the nerve terminal. We are fascinated by how cytosol remains soluble to allow metabolic processes to occur concomitantly in space and time. The goal of Milovanovic Lab is to understand: How is the spatial organization of organelles and macromolecules regulated in the crowded environment of the cytosol at the nerve terminal?
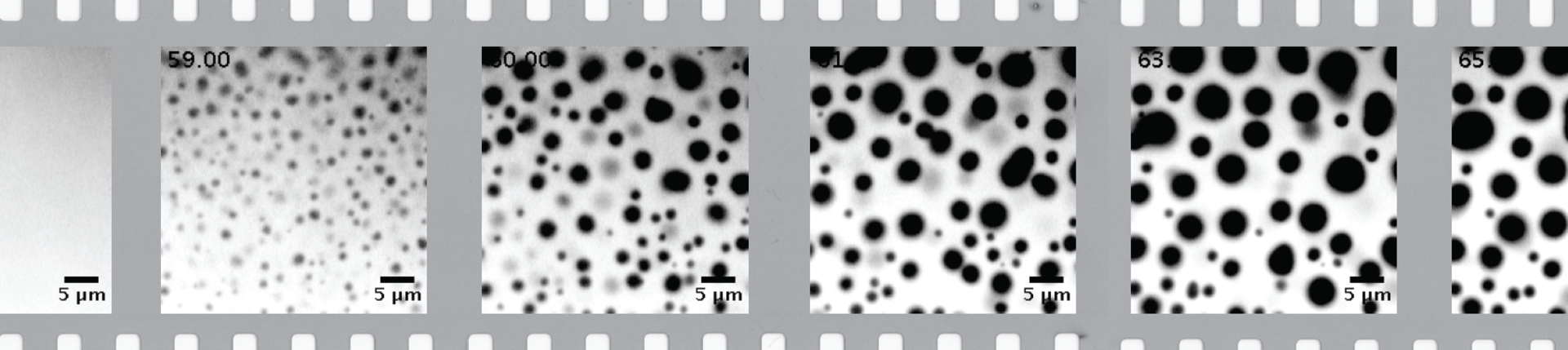
We hypothesize that the presence of intrinsically disordered regions (IDRs)–low amino acid complexity sequences that do not fold into any stable secondary or tertiary structure—plays an essential role in balancing the solubility with the spatial patterning. Many proteins involved in the synaptic vesicle recycling contain long IDRs. Recently, it was shown that synapsin 1, a highly abundant synaptic protein, forms a distinct liquid phase in an aqueous environment (Fig. 1) (Milovanovic et al., 2018; Hoffmann et al., 2023). Synapsin condensates are reaction centers able to sequester lipid vesicles. This mechanism helped to explain how hundreds of SVs are kept into distinct clusters while maintaining their high mobility (Milovanovic and De Camilli, 2017; Sansevrino et al., 2023).
Significance. Given the central roles of proteins with IDRs in the pathology of many neurodegenerative diseases, our work will open new diagnostic and therapeutic strategies to combat these diseases. For example, a-synuclein is involved in a family of diseases that include Parkinson's and Dementia with Lewy Bodies and contains an IDR. Tau, another neuronal protein that contains an IDR, is associated with Alzheimer's disease. Neuronal RNA-binding proteins such as hnRNPA1, FUS, TDP43 are all linked to Amyotrophic Lateral Sclerosis and Frontotemporal Dementia and contain IDRs. Classically, the hallmark of the abovementioned neurodegenerative diseases is misfolding and aggregation of these proteins/their IDRs. Strikingly, the recent analysis of human postmortem brain tissues of Parkinson's patients shows that aggregates are, in fact, a crowded medley of vesicular structures. Hence, it is of crucial importance to understand the underlying mechanisms that keep these central organellar components soluble and functional in the crowded environment of the nerve terminal.
NeuroPhaseClinic (NPC) is a think-tank put forward by the Milovanovic Lab to foster the exchange across disciplines from (bio)chemistry, physics, engineering, and medicine for curing neurodegenerative diseases. We also aim to share the knowledge and the latest developments among all stakeholders - researchers, patients, and caregivers.
The NPC is organizing a series of seminars and round-tables:
Guest | Affiliation | When? | Where? | Title |
|---|---|---|---|---|
| Dr. Ulf Dettmer | Brigham and Women’s Hospital (Harvard Medical School) | 01‑03‑2024 | Charité CrossOver Berlin, DE | The dynamic cellular biology of α‑synuclein. |
| Dr. Shigeo Takamori | Doshisha University in Kyoto/Japan | 18‑03‑2024 | Charité CrossOver Berlin, DE | Mechanism of Neurotransmitter Uptake into Synaptic Vesicles: Perspectives from Proton Imaging. |
| Dr. Thierry Galli | Institute of Psychiatry and Neuroscience of Paris | 19‑04‑2024 | Charité CrossOver Berlin, DE | Molecular and cellular mechanisms of late endosomal autophagy-dependent unconventional secretion - importance in glioblastoma. |
| Dr. Zach Geisterfer | Duke University | 13‑05‑2024 | Charité CrossOver Berlin, DE | Condensates enable complex cell morphology through localized translation. |
| Dr. Sylwia Kierszniowska / Dr. Nikola Milosevic | MetaSysX GmbH / | 14‑06‑2024 | Charité CrossOver Berlin, DE | The emerging roles of AI in contemporary research design and discovery: an industry perspective. |
| Dr. Vincent Boudreau | Director of Scientific Research, | 04‑07‑2024 | MBL Woods Hole, US | Battling Climate Change:The Frontline Roles of Cell Biologists. |
| Dr. Julie Klaric | Development Scientist, | 08‑07‑2024 | MBL Woods Hole, US | Navigating Continents and Careers: Making the Leap from Academia to Industry. |
| Dr. Lara Szewczak | Deputy Editor, | 10‑07‑2024 | MBL Woods Hole, US | Charting the Future of Scientific Publishing. |
| Dr. Simon Bertrand | Senior Scientist, | 19‑07‑2024 | Charité CrossOver Berlin, DE | From Postdoc to Startup: Charting the Course in European Life Science |
| Dr. Elva Diaz | UC Davis | 16‑09‑2024 | Charité CrossOver Berlin, DE | Molecular mechanisms of excitatory synapse development, plasticity and disease |




