Research areas/focus
The long-standing focus of our research team has been on mechanisms of neurodegeneration and alpha-synuclein toxicity in Parkinson’s disease (PD). We have developed and characterized a variety of experimental models mimicking specific aspects of PD pathology and, for our work, we apply state-of-the-art histological and molecular biology techniques. Through these tools, we are investigating:
- the role of specific mechanisms, such as mitochondrial failure, protein degradation impairment, microglial activation and neuron-to-neuron protein transfer, in the pathogenesis of neurodegeneration and alpha-synuclein accumulation/aggregation in PD;
- how disease risk factors, such as aging, genetic variants and environmental exposures, can influence the onset and progression of PD pathology, and
- the effectiveness of new therapeutic strategies (e.g., RNA silencing, immunotherapy and regenerative cell therapy) in preventing, slowing down or halting the pathological process underlying PD.
Although our research is mainly focused on PD, we are studying mechanisms and risk factors that are shared between PD and other neurodegenerative disorders. We believe that bridging different diseases will help us address key pathogenetic questions concerning, for example, mechanisms of selective neuronal vulnerability and conditions leading to clinical and pathological co-morbidity.
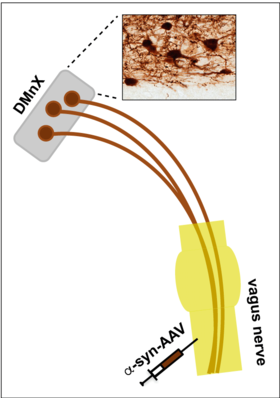
Experimental Models. Figure 1 illustrates a strategy developed in our laboratory aimed at inducing targeted overexpression of alpha-synuclein in the dorsal motor nucleus of the vagus nerve (DMnX). Viral vectors (AAV) carrying alpha-synuclein DNA are injected into the vagus nerve and, traveling via fibers of this nerve, reach and transduce DMnX neurons. DMnX neurons, shown in the histological image, are among the cells affected early by alpha-synuclein pathology during PD development.
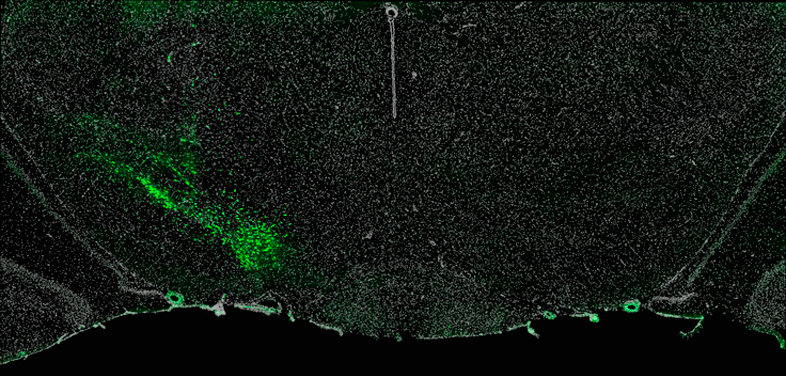
Techniques. Figure 2 shows neurons in the mouse substantia nigra pars compacta labeled (green) for alpha-synuclein mRNA by fluorescent in situ hybridization. The substantia nigra is a primary target of PD neurodegenerative processes, and alpha-synuclein is a key protein involved in PD pathology.
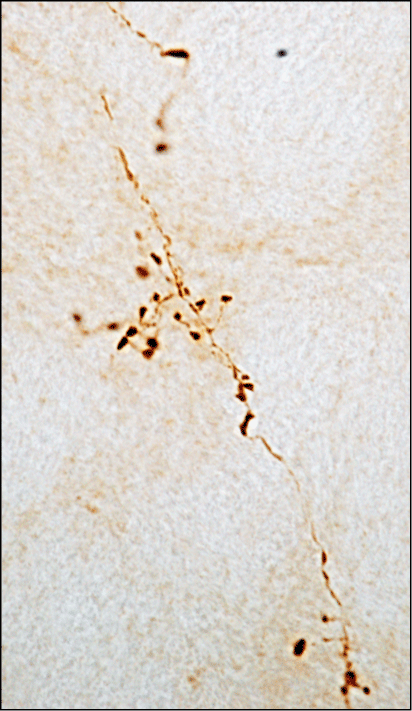
Mechanisms. Figure 3 shows axonal projections in the rat locus coeruleus that are loaded with alpha-synuclein as a result of protein spreading from caudal (medulla oblongata) to rostral (pons) brain regions. The locus coeruleus is a pontine nucleus particularly vulnerable to neurodegeneration and alpha-synuclein pathology in PD. Alpha-synuclein accumulation as a consequence of protein spreading could play a role in this enhanced vulnerability.
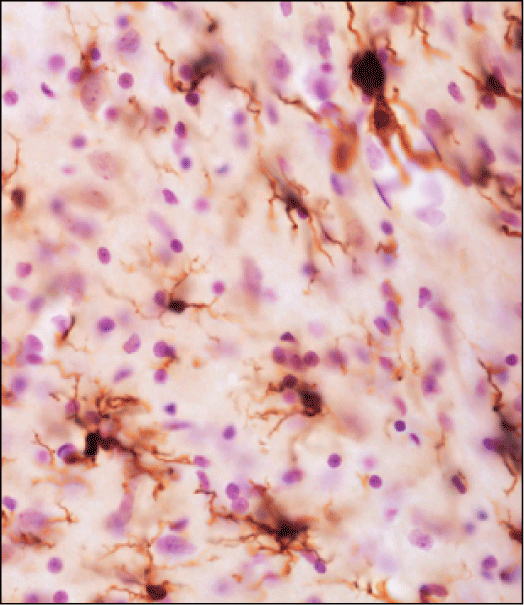
Risk Factors. Figure 4 shows activated microglial cells in the locus coeruleus of aging rats as a consequence of caudo (medulla oblongata)-rostral (pons) alpha-synuclein spreading. Microglial activation could render the aging brain highly susceptible to neurodegenerative processes.
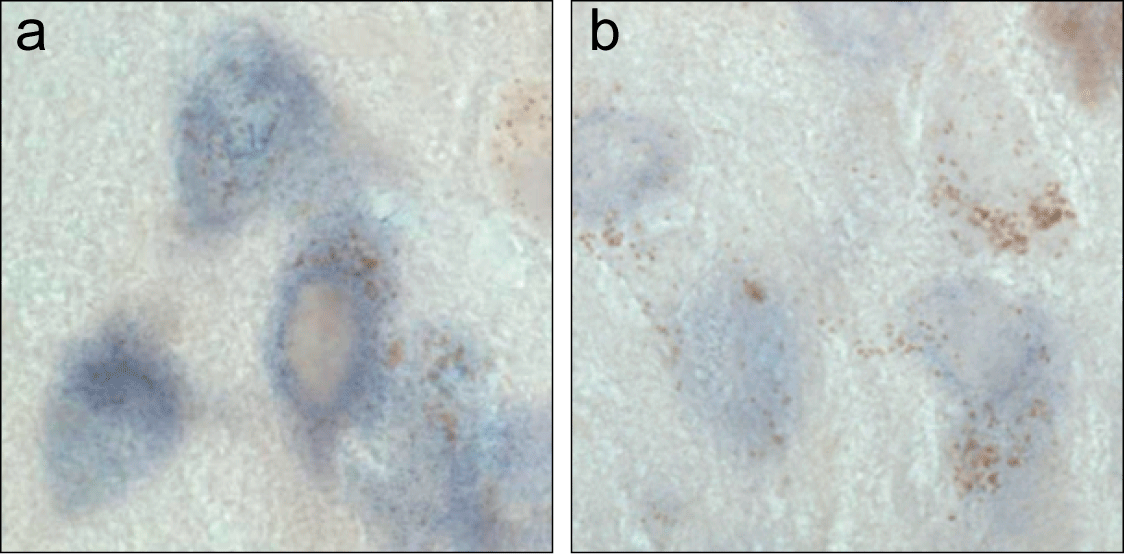
Therapeutic Strategies. Figure 5 shows substantia nigra neurons labeled (purple) for alpha-synuclein mRNA by in situ hybridization. A comparison is made between normal neurons (a) vs. neurons treated with small interfering RNA against alpha-synuclein (b). Decreased purple labeling in (b) indicates reduced alpha-synuclein expression and suggests that RNA interference may be used to protect neurons from alpha-synuclein toxicity.