Clinical Research Platform successfully recertified
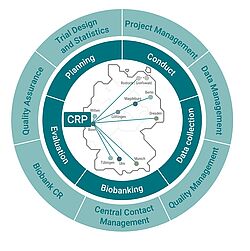
The Clinical Research Platform (CRP) at the Centre for Clinical Research in Bonn supports and coordinates the multi-site studies of the DZNE. Within the clinical research of the DZNE, the CRP provides an organizational framework for the planning, execution and evaluation of clinical trials as well as the collection of all data and biobanking under standardized conditions. The CRP comprises the areas of project management, data management, trial design and statistics, central contact management, clinical research biorepository, quality assurance and quality management. The study database is operated in cooperation with DZNE-IT. The composition of the CRP team of experienced scientific, clinical and industrial staff ensures a high degree of quality, transparency and consistency of clinical studies from conception to completion
In 2013, CRP has started to introduce a quality management system according to DIN EN ISO 9001:2015. This was certified for the first time in September 2017 and successfully recertified with effective date September 11, 2020.